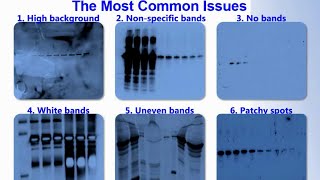
Western Blot troubleshooting involves addressing issues like weak signals or background noise. Ensure proper sample preparation and correct antibody dilutions.
Western Blotting is a crucial technique in molecular biology for protein detection and analysis. It provides information on protein expression, size, and abundance. Despite its importance, researchers often face challenges with Western Blotting. Common issues include weak or non-specific signals, high background noise, and inconsistent results.
Addressing these problems requires a systematic approach. Proper sample preparation, accurate antibody selection, and optimized blotting conditions are key. Regularly checking reagents and equipment can also prevent issues. By understanding common pitfalls and implementing best practices, researchers can achieve reliable and reproducible Western Blot results.
Credit: www.youtube.com
Introduction To Western Blot
Western Blot is a widely-used technique in molecular biology. It helps in detecting specific proteins in a sample. This method is essential for various research and diagnostic applications.
Purpose And Applications
Western Blot serves several purposes in scientific research. It helps identify and quantify specific proteins. This method is vital for confirming gene expression. Researchers use it to study protein-protein interactions.
Applications of Western Blot are extensive. It is used in medical diagnostics to detect diseases. Scientists use it in drug development and biomarker discovery. It plays a crucial role in cancer research.
Basic Principles
The basic principle of Western Blot involves several steps. First, proteins are separated by gel electrophoresis. Then, they are transferred to a membrane. This membrane is usually made of nitrocellulose or PVDF.
Next, the membrane is incubated with primary antibodies. These antibodies bind to the target protein. After washing, secondary antibodies are added. These are linked to a detection enzyme or fluorescent tag.
Finally, the signal is detected using various methods. Chemiluminescence and fluorescence are common detection methods. The intensity of the signal indicates the amount of target protein.
| Step | Description |
|---|---|
| Gel Electrophoresis | Separates proteins based on size |
| Transfer | Moves proteins to a membrane |
| Primary Antibody Incubation | Antibodies bind to target proteins |
| Secondary Antibody Incubation | Antibodies linked to detection tags |
| Detection | Signal indicates protein amount |
Common Issues
Western blotting is a powerful technique for protein analysis. Yet, it often comes with challenges. In this section, we will discuss some common issues. You will learn how to handle weak signals and high background.
Weak Signals
Weak signals can make your results hard to interpret. Here are some common causes and solutions:
- Insufficient Protein Loading: Ensure you load enough protein in the gel. Aim for 20-30 µg per lane.
- Antibody Concentration: Check your primary and secondary antibody concentrations. You may need to optimize them.
- Transfer Efficiency: Verify that proteins efficiently transferred from gel to membrane. Use Ponceau S staining to confirm transfer.
- Detection Reagents: Ensure your detection reagents are fresh and working properly. This includes substrates and chemiluminescent solutions.
High Background
High background can obscure specific signals. This makes interpretation difficult. Consider the following tips:
- Blocking Buffer: Use an effective blocking buffer. Common choices include 5% BSA or non-fat dry milk in TBS-T.
- Washing Steps: Perform multiple washes after antibody incubations. Use TBS-T for washing.
- Antibody Specificity: Verify that your antibodies are specific to your target. Non-specific antibodies can increase background.
- Incubation Conditions: Optimize incubation times and temperatures. Too long or too high can increase background.
Sample Preparation Tips
Proper sample preparation is crucial for successful Western blotting. Many problems can arise from poor preparation. Below are essential tips to ensure high-quality samples.
Lysis Buffer Choice
Choosing the right lysis buffer is vital. It ensures efficient protein extraction. Different cells and tissues need different buffers. Here are some common lysis buffers:
| Buffer Type | Usage |
|---|---|
| RIPA Buffer | For most cell types |
| NP-40 Buffer | For nuclear proteins |
| CHAPS Buffer | For membrane proteins |
Always include protease inhibitors in your buffer. This prevents protein degradation.
Protein Quantification
Accurate protein quantification is essential. It ensures equal loading and reproducibility. Here are some steps for quantification:
- Use a BCA Assay for accurate results.
- Prepare a standard curve using known protein concentrations.
- Measure the absorbance of your samples.
- Compare sample absorbance to the standard curve.
Load equal amounts of protein for each sample. This ensures consistent results.
Label your samples clearly. This avoids mix-ups and errors during loading.

Credit: www.youtube.com
Gel Electrophoresis Solutions
Western blotting is a powerful technique for protein analysis. But, gel electrophoresis can present challenges. This section will help troubleshoot common issues with gel electrophoresis. Focus on proper gel selection and loading control.
Proper Gel Selection
Choosing the right gel is crucial for successful Western blotting. There are different types of gels for different proteins.
Polyacrylamide gels are popular. They separate proteins by size. Use lower percentage gels for larger proteins. Use higher percentage gels for smaller proteins.
Gradient gels have varying concentrations. They are great for separating a range of protein sizes. Choose a gradient gel if your sample has mixed sizes.
Make sure to check the gel’s expiration date. Old gels can cause poor separation.
Loading Control
Loading control ensures equal protein amounts are loaded in each lane. This is important for accurate results.
Use a loading control antibody. It detects a protein that is consistently expressed. Common loading controls are actin, GAPDH, and tubulin.
- Actin: Good for most cell types.
- GAPDH: Works well in many conditions.
- Tubulin: Ideal for cytoskeletal studies.
Run a pilot experiment to validate your loading control. This ensures it is suitable for your samples.
| Protein | Optimal Gel Percentage |
|---|---|
| Small proteins (<20 kDa) | 15-20% |
| Medium proteins (20-100 kDa) | 10-15% |
| Large proteins (>100 kDa) | 5-10% |
Use the right gel and loading control for reliable results. Proper selection and validation are key.
Transfer Efficiency
Transfer efficiency is crucial in Western Blotting. Poor transfer can result in weak or absent bands. Proper transfer ensures your proteins move from the gel to the membrane. This section focuses on how to improve transfer efficiency.
Membrane Selection
Choosing the right membrane is key for good transfer. There are two main types: nitrocellulose and PVDF.
- Nitrocellulose: Good for proteins under 100 kDa. It has a high binding capacity.
- PVDF: Better for larger proteins. It is durable and can be stripped and re-probed.
Make sure the membrane suits your protein size. This will enhance transfer efficiency.
Transfer Conditions
Transfer conditions affect efficiency greatly. Key factors include voltage, time, and buffer composition.
Voltage: Use low voltage for longer transfers. Use high voltage for quicker transfers.
Time: Longer transfer times increase efficiency. This is especially true for large proteins.
Buffer Composition: Use fresh buffer. Ensure correct pH and ionic strength.
Here is a quick reference table for transfer conditions:
| Protein Size | Voltage | Time | Buffer |
|---|---|---|---|
| Small (< 30 kDa) | 100V | 1 hour | Standard Transfer Buffer |
| Medium (30-80 kDa) | 75V | 2 hours | Standard Transfer Buffer |
| Large (> 80 kDa) | 50V | Overnight | Standard Transfer Buffer with Methanol |
Adjust these parameters based on your protein and membrane type. This will help you achieve optimal transfer efficiency.
Blocking And Antibody Optimization
Optimizing your Western Blot can be challenging. Blocking and antibody optimization are key steps. Getting these steps right ensures clear, reliable results.
Blocking Reagents
Blocking reagents prevent nonspecific binding. Common reagents include BSA, milk, and commercial blockers.
| Reagent | Concentration | Benefits |
|---|---|---|
| Bovine Serum Albumin (BSA) | 1-5% | Reduces background noise |
| Non-fat Dry Milk | 3-5% | Cost-effective and widely used |
| Commercial Blockers | Follow manufacturer’s instructions | Optimized formulations |
Primary And Secondary Antibodies
Primary antibodies bind to your target protein. Secondary antibodies bind to the primary antibody.
Use the correct antibody concentration. High concentrations can increase background noise. Low concentrations can reduce signal strength.
- Primary Antibody: Dilute in blocking buffer. Typical range: 1:500 to 1:5000.
- Secondary Antibody: Dilute in blocking buffer. Typical range: 1:1000 to 1:20000.
Optimize incubation times. Primary antibody incubation typically lasts overnight at 4°C. Secondary antibody incubation usually lasts 1 hour at room temperature.
Detection Techniques
Western blot detection techniques are crucial for accurate protein analysis. There are two primary detection methods: chemiluminescence and fluorescence. Each method has its unique advantages and applications.
Chemiluminescence
Chemiluminescence is a popular detection technique in Western blotting. It relies on enzyme-linked antibodies. These enzymes catalyze reactions that emit light. This light is then captured on photographic film or digital imaging systems.
- Advantages:
- High sensitivity
- Cost-effective
- Wide dynamic range
- Disadvantages:
- Short signal duration
- Potential for non-specific binding
To achieve the best results, use fresh reagents. Optimize antibody concentrations. Ensure proper exposure times.
Fluorescence
Fluorescence detection involves fluorophore-labeled antibodies. These antibodies emit light at specific wavelengths. The emitted light is detected by a fluorescence imaging system.
- Advantages:
- High signal stability
- Multiplexing capabilities
- Quantitative accuracy
- Disadvantages:
- Higher cost
- Requires specialized equipment
For optimal fluorescence results, use high-quality fluorophores. Ensure proper excitation and emission settings. Avoid overlapping spectra in multiplex experiments.
| Detection Method | Advantages | Disadvantages |
|---|---|---|
| Chemiluminescence | High sensitivity, Cost-effective, Wide dynamic range | Short signal duration, Non-specific binding |
| Fluorescence | High signal stability, Multiplexing, Quantitative accuracy | Higher cost, Specialized equipment |
Data Analysis And Interpretation
Western blotting is a powerful tool for protein detection. But, analyzing the data can be tricky. This section will help you understand how to interpret your results. We will cover quantifying bands and normalization methods.
Quantifying Bands
Quantifying bands is a crucial part of data analysis. It involves measuring the intensity of protein bands on a blot. There are various software tools available to assist with this.
Use the following steps to quantify bands accurately:
- Load your image into the software.
- Select the bands you want to measure.
- Adjust the background settings to remove noise.
- Measure the intensity of each band.
Keep a record of your measurements. This helps in comparing the results later. Here is an example table to organize your data:
| Sample | Band Intensity |
|---|---|
| Sample 1 | 150 |
| Sample 2 | 200 |
Normalization Methods
Normalization methods are essential for accurate data interpretation. These methods adjust for variations in sample loading and transfer efficiency.
Common normalization methods include:
- Housekeeping Proteins: Proteins like GAPDH or β-Actin are used.
- Total Protein Staining: Stain the entire blot to measure total protein.
- Loading Controls: Use a control protein to normalize your data.
To normalize your data, follow these steps:
- Measure the intensity of the housekeeping protein or control.
- Divide the band intensity of your target protein by the housekeeping protein intensity.
- Record the normalized values.
Normalization helps in getting accurate and reliable results. Always use appropriate controls for your experiments. This ensures your data is trustworthy.
Advanced Troubleshooting Tips
Western blotting is a powerful tool. Sometimes, unexpected issues can arise. Advanced troubleshooting can save time and resources. Below are some tips to tackle common problems.
Unexpected Bands
Unexpected bands can confuse results. These bands may occur due to non-specific binding. Non-specific binding often results from antibodies binding to unintended targets.
- Use pre-absorbed antibodies to reduce non-specific binding.
- Include a blocking step with appropriate buffers.
- Optimize antibody concentration to avoid excess binding.
Cross-reactivity can also cause unexpected bands. Cross-reactivity happens when antibodies bind similar proteins.
- Use highly specific antibodies to minimize cross-reactivity.
- Check the manufacturer’s datasheet for known cross-reactivities.
Reproducibility Issues
Reproducibility is crucial in western blotting. Inconsistent results can be frustrating. Here are some tips to improve reproducibility.
- Ensure consistent sample preparation to avoid variability.
- Use the same gel concentration for all experiments.
- Maintain consistent transfer conditions between blots.
Reproducibility can also be affected by antibody variability. Different antibody batches may vary in performance.
- Validate each new batch of antibodies before use.
- Store antibodies under recommended conditions to maintain efficacy.
Using a control sample can help track variability. Always include a positive control in your experiments.
| Issue | Solution |
|---|---|
| Non-specific binding | Use pre-absorbed antibodies |
| Cross-reactivity | Check datasheet for cross-reactivities |
| Sample variability | Ensure consistent sample preparation |
| Antibody variability | Validate new antibody batches |

Credit: www.researchgate.net
Frequently Asked Questions
What Are The Common Errors Encountered In Western Blotting?
Common errors in Western blotting include weak signals, background noise, non-specific bands, uneven transfer, and antibody cross-reactivity.
What Is The Source Of Error In Western Blot?
Errors in Western blot can arise from sample preparation, antibody specificity, transfer efficiency, or detection method. Proper controls and optimization minimize these issues.
What Can Cause A False Positive Western Blot?
Contaminated samples, cross-reactivity, improper washing, and overexposure of the film can cause false positive Western blot results.
What Are The Spots On My Western Blot?
Spots on your Western blot can result from non-specific binding, uneven transfer, or contaminated reagents. Ensure proper blocking, clean equipment, and use fresh buffers to reduce spots.
Conclusion
Mastering Western blot troubleshooting can save time and resources. Follow these tips for consistent and reliable results. Practice makes perfect, so keep experimenting. Your confidence will grow with each successful blot. Troubleshooting is an essential skill for any lab professional.
Stay patient and persistent for the best outcomes.